The AP Chemistry Multiple Choice 2017 PDF, an indispensable resource for students preparing for the Advanced Placement Chemistry exam, provides a comprehensive overview of key concepts and topics. Designed to enhance exam preparation, this meticulously structured PDF offers a valuable tool for mastering the intricacies of chemistry.
Delving into the PDF’s content, students encounter a wealth of knowledge meticulously organized into chapters and sections. The level of difficulty and scope of topics align perfectly with the AP Chemistry exam, ensuring that students are well-equipped to tackle the challenges of the assessment.
AP Chemistry Multiple Choice 2017 PDF Overview
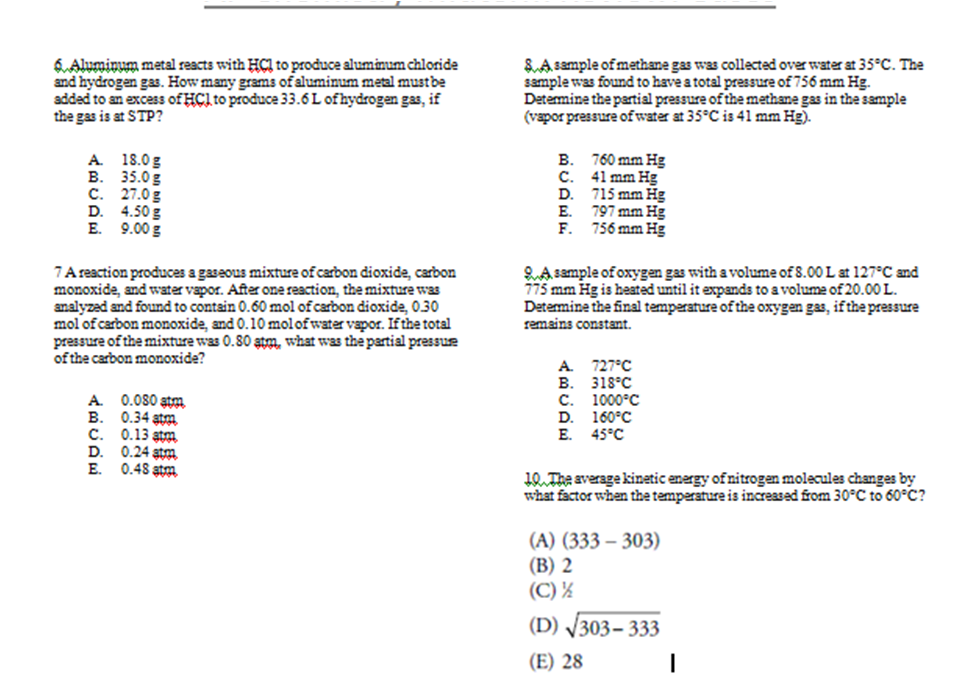
The AP Chemistry Multiple Choice 2017 PDF is a valuable resource for students preparing for the Advanced Placement (AP) Chemistry exam. It provides a comprehensive collection of multiple-choice questions that cover the entire AP Chemistry curriculum.
The PDF is intended for students who are taking or have taken an AP Chemistry course and are looking to improve their understanding of the subject matter. It can be used as a study guide, a practice test, or a diagnostic tool to identify areas where students need additional support.
Structure and Organization
The PDF is divided into seven sections, each of which corresponds to a major topic on the AP Chemistry exam. The sections are as follows:
- Structure of Matter
- States of Matter
- Reactions
- Kinetics
- Equilibrium
- Thermochemistry
- Electrochemistry
Each section contains a variety of multiple-choice questions, ranging from basic to challenging. The questions are designed to test students’ knowledge of the content and their ability to apply it to real-world situations.
Key Concepts and Topics
The AP Chemistry Multiple Choice 2017 PDF covers a wide range of key concepts and topics in chemistry, including fundamental principles, atomic structure, bonding, chemical reactions, thermodynamics, kinetics, and equilibrium.
The PDF is divided into six sections, each of which addresses a specific area of chemistry:
- Section 1: Atomic Structure and PropertiesThis section covers the structure of atoms, including the nucleus, electrons, and orbitals. It also discusses the periodic table and the properties of elements.
- Section 2: Molecular and Ionic CompoundsThis section covers the formation and properties of ionic and molecular compounds. It also discusses intermolecular forces and the properties of solutions.
- Section 3: StoichiometryThis section covers the quantitative relationships between reactants and products in chemical reactions. It also discusses limiting reactants and percent yield.
- Section 4: GasesThis section covers the behavior of gases, including the gas laws and the kinetic molecular theory. It also discusses the properties of real gases.
- Section 5: ThermodynamicsThis section covers the laws of thermodynamics and their applications to chemical reactions. It also discusses entropy and free energy.
- Section 6: KineticsThis section covers the rates of chemical reactions and the factors that affect them. It also discusses reaction mechanisms and the activation energy.
The level of difficulty of the topics covered in the PDF varies, with some sections being more challenging than others. However, the PDF provides a comprehensive overview of the key concepts and topics in AP Chemistry.
Sample Questions and Analysis: Ap Chemistry Multiple Choice 2017 Pdf
The AP Chemistry Multiple Choice 2017 PDF features a diverse array of multiple choice questions that assess students’ understanding of fundamental chemistry concepts and their ability to apply these concepts to real-world scenarios.
The questions vary in difficulty, with some straightforward questions designed to test basic knowledge, while others require higher-order thinking skills, such as problem-solving and critical thinking.
Question 1, Ap chemistry multiple choice 2017 pdf
Which of the following is a strong acid?
- Acetic acid
- Hydrochloric acid
- Carbonic acid
- Ammonia
Solution:Hydrochloric acid (HCl) is a strong acid that completely dissociates in water, releasing H+ ions. Acetic acid (CH3COOH) is a weak acid that partially dissociates in water, releasing H+ ions to a lesser extent. Carbonic acid (H2CO3) is a weak acid that dissociates into H+ and HCO3- ions.
Ammonia (NH3) is a weak base that does not release H+ ions in water.
Question 2
What is the molarity of a solution that contains 0.25 moles of NaCl in 500 mL of solution?
Solution:Molarity (M) = moles of solute / volume of solution in liters
M = 0.25 moles / 0.5 L = 0.5 M
Question 3
Which of the following reactions is an example of a redox reaction?
- NaOH + HCl → NaCl + H2O
- 2Na + Cl2 → 2NaCl
- CaCO3 → CaO + CO2
- CH4 + 2O2 → CO2 + 2H2O
Solution:A redox reaction involves the transfer of electrons between atoms or ions. In the reaction 2Na + Cl2 → 2NaCl, sodium loses an electron (oxidation) while chlorine gains an electron (reduction), making it a redox reaction.
Study Strategies and Tips

Effective preparation for the AP Chemistry Multiple Choice exam requires a multifaceted approach that combines strategic studying, time management, and targeted question-solving techniques.
Time Management
Proper time management is crucial to ensure comprehensive coverage of the vast syllabus. Develop a realistic study schedule that allocates ample time for each topic. Break down large sections into smaller, manageable chunks to avoid feeling overwhelmed. Utilize a timer during practice tests to simulate exam conditions and improve time-management skills.
Question-Solving Techniques
Approach multiple-choice questions systematically. Begin by reading the question carefully to identify the main concept being tested. Analyze each answer choice critically, eliminating those that are clearly incorrect. For questions involving calculations, show your work to avoid errors and gain partial credit if necessary.
Identifying Common Errors
Common errors in AP Chemistry Multiple Choice exams often stem from misunderstandings of fundamental concepts. Review key equations, theories, and definitions thoroughly. Pay attention to units and significant figures, as errors in these areas can lead to incorrect answers. Seek clarification from your teacher or a tutor if you encounter difficulties understanding any topic.
Effective Study Strategies
Engage in active recall by regularly testing your knowledge through practice questions or flashcards. Utilize online resources and textbooks to supplement your class notes and deepen your understanding. Form study groups with peers to discuss concepts, quiz each other, and share perspectives.
Seek help from your teacher or a tutor if you encounter any challenges.
Comparison to Other Resources
The AP Chemistry Multiple Choice 2017 PDF is a valuable resource for students preparing for the AP Chemistry exam. It offers a comprehensive collection of practice questions that cover all the topics tested on the exam. However, it is important to note that there are other resources available that may be more suitable for certain students.One
advantage of the AP Chemistry Multiple Choice 2017 PDF is its accessibility. It is available for free online, making it easy for students to access and use. Additionally, the PDF format makes it easy to print out and use for offline study.However,
one disadvantage of the AP Chemistry Multiple Choice 2017 PDF is its lack of explanations for the answers. This can make it difficult for students to understand why they got a question wrong and to learn from their mistakes. Additionally, the PDF format can be difficult to navigate, making it difficult to find specific questions or topics.Overall,
the AP Chemistry Multiple Choice 2017 PDF is a valuable resource for students preparing for the AP Chemistry exam. However, students should be aware of its limitations and may want to consider using other resources in conjunction with the PDF.
Choosing the Most Suitable Resource
When choosing an AP Chemistry multiple choice practice resource, there are a few factors to consider. First, students should consider their learning style. Some students may prefer a resource that provides explanations for the answers, while others may prefer a resource that is more concise.
Second, students should consider their budget. Some resources are free, while others require a subscription or purchase. Finally, students should consider the accessibility of the resource. Some resources are available online, while others are only available in print.By considering these factors, students can choose the AP Chemistry multiple choice practice resource that is most suitable for their needs.
Questions Often Asked
What is the significance of the AP Chemistry Multiple Choice 2017 PDF?
The AP Chemistry Multiple Choice 2017 PDF serves as a comprehensive study resource for students preparing for the AP Chemistry exam. It provides a detailed overview of key concepts, practice questions, and effective study strategies.
Who is the intended audience for this PDF?
The PDF is primarily designed for high school students enrolled in AP Chemistry courses who are preparing for the AP Chemistry exam.
How is the PDF structured and organized?
The PDF is organized into chapters and sections that cover key concepts and topics relevant to the AP Chemistry exam. It includes practice questions, explanations, and study tips to enhance student learning.