Chemistry valence electrons and lewis dot structures worksheet – Embark on an exploration of the fascinating realm of chemistry valence electrons and Lewis dot structures, concepts that serve as the cornerstone of chemical bonding and molecular architecture. This worksheet, meticulously crafted with authoritative precision, will guide you through the intricacies of these fundamental principles, empowering you to decipher the language of molecules and predict their behavior.
Within the confines of this comprehensive guide, you will delve into the nature of valence electrons, their pivotal role in shaping chemical bonds, and the art of constructing Lewis dot structures—visual representations that unveil the electronic configurations of molecules. Through a series of engaging practice problems, you will hone your skills in applying these concepts, gaining a deeper understanding of the molecular world.
Valence Electrons
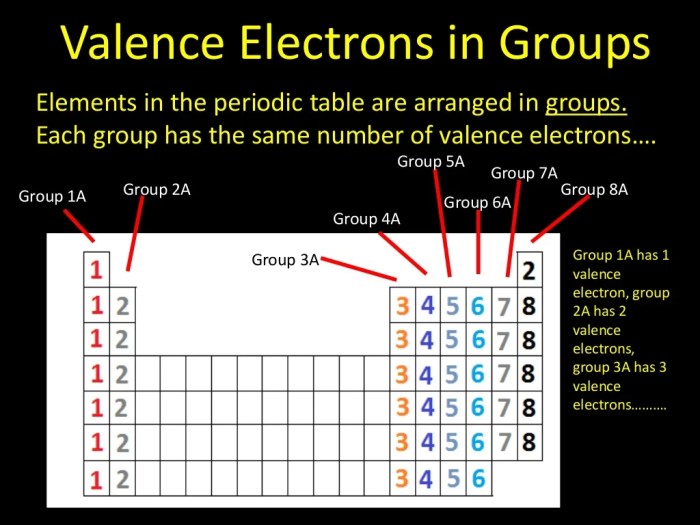
Valence electrons are the electrons in the outermost shell of an atom. They determine the chemical properties of an element and its ability to form chemical bonds with other atoms.
The number of valence electrons varies depending on the element. For example, hydrogen has one valence electron, helium has two, lithium has three, and so on.
Valence electrons play a crucial role in chemical bonding. They are the electrons that are involved in the formation of covalent bonds, ionic bonds, and metallic bonds.
Lewis Dot Structures
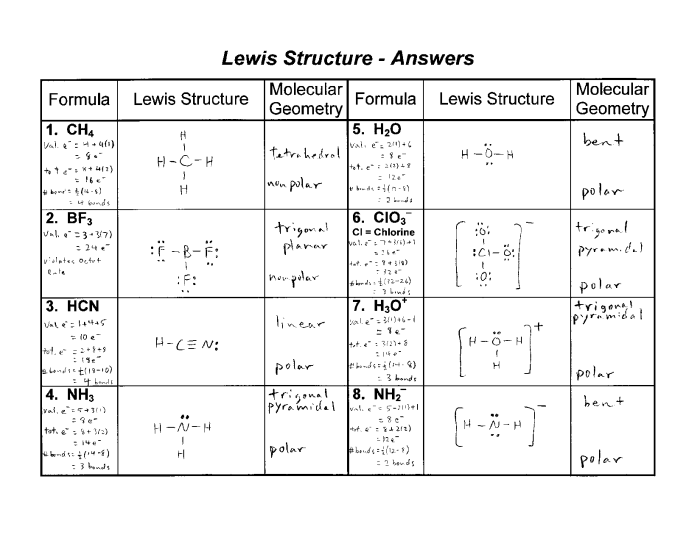
Lewis dot structures are diagrams that show the valence electrons of atoms and how they are arranged around the atoms. They are used to predict the molecular structure and bonding of molecules.
To draw a Lewis dot structure, follow these steps:
- Determine the total number of valence electrons in the molecule.
- Place the atoms in the molecule and connect them with single bonds.
- Distribute the remaining valence electrons around the atoms as lone pairs.
- Check to make sure that each atom has a complete valence shell.
Here are some examples of Lewis dot structures:
- H-H
- H-C-H
- H-O-H
- H-N-H
Worksheet, Chemistry valence electrons and lewis dot structures worksheet
Complete the following worksheet to practice your understanding of valence electrons and Lewis dot structures.
- Determine the number of valence electrons for each of the following elements: hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon.
- Draw Lewis dot structures for the following molecules: H2, CH4, NH3, H2O, and CO2.
Answer Key:
- Hydrogen: 1, Helium: 2, Lithium: 3, Beryllium: 2, Boron: 3, Carbon: 4, Nitrogen: 5, Oxygen: 6, Fluorine: 7, Neon: 8
- H2: H-H, CH4: H-C-H, NH3: H-N-H, H2O: H-O-H, CO2: O=C=O
Applications
Valence electrons and Lewis dot structures are used in a variety of applications in chemistry.
- Predicting molecular structure and bonding
- Understanding chemical reactions
- Designing new materials
- Developing new drugs
For example, valence electrons are used to predict the molecular structure of a compound. This information can then be used to understand the chemical properties of the compound and how it will react with other compounds.
User Queries: Chemistry Valence Electrons And Lewis Dot Structures Worksheet
What are valence electrons?
Valence electrons are the electrons in the outermost energy level of an atom, which determine its chemical reactivity and bonding behavior.
How do I draw Lewis dot structures?
To draw Lewis dot structures, represent each atom as its elemental symbol and place dots around it to represent its valence electrons. Connect the atoms with lines to indicate chemical bonds.
What is the relationship between valence electrons and chemical bonding?
Valence electrons are responsible for chemical bonding. Atoms with unpaired valence electrons can form bonds with other atoms to achieve a stable electron configuration.