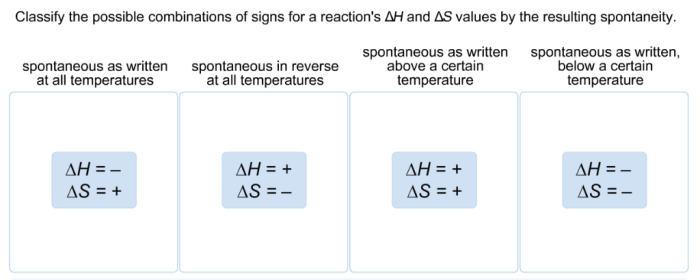
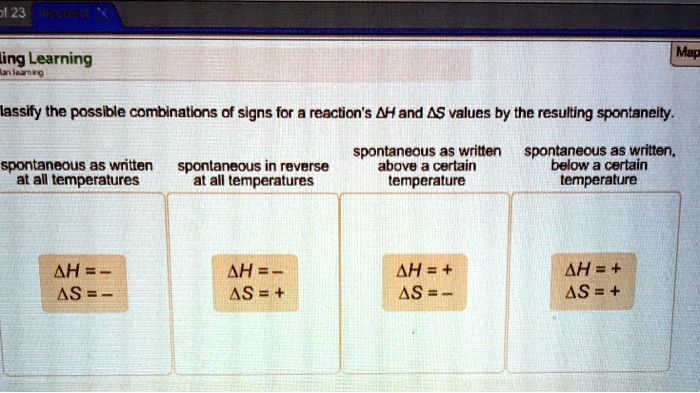
Classify the possible combinations of signs for a reactions – Delving into the realm of classifying the possible combinations of signs for reactions, this exploration unveils a captivating tapestry of scientific inquiry, where the intricate interplay of signs and reactions holds profound implications for our understanding of chemical processes. As we embark on this journey, we unravel the significance of deciphering sign combinations, revealing their pivotal role in predicting and comprehending the behavior of chemical reactions.
Introduction to Signs and Reactions
Signs and reactions are fundamental concepts in chemistry that describe the behavior of chemical species in reactions. A sign refers to the positive or negative charge of an ion, while a reaction is a chemical change that involves the transformation of reactants into products.
Classifying sign combinations for reactions is crucial for understanding and predicting the outcome of chemical reactions.
Methods for Classifying Sign Combinations
There are several approaches to classifying sign combinations for reactions:
- Based on the sign of the reactants and products:This method classifies reactions as positive-positive (both reactants and products are positive), negative-negative (both reactants and products are negative), positive-negative (reactants are positive and products are negative), or negative-positive (reactants are negative and products are positive).
- Based on the sign of the overall reaction:This method classifies reactions as exothermic (overall sign is negative, energy is released) or endothermic (overall sign is positive, energy is absorbed).
- Based on the sign of the equilibrium constant:This method classifies reactions as spontaneous (equilibrium constant is positive) or nonspontaneous (equilibrium constant is negative).
Types of Sign Combinations
The different types of sign combinations for reactions include:
- Positive-positive:Both reactants and products are positive, indicating a net release of energy.
- Negative-negative:Both reactants and products are negative, indicating a net absorption of energy.
- Positive-negative:Reactants are positive and products are negative, indicating a net decrease in energy.
- Negative-positive:Reactants are negative and products are positive, indicating a net increase in energy.
Applications of Sign Combination Classification
Classifying sign combinations for reactions has several practical applications:
- Predicting the spontaneity of reactions:The sign of the overall reaction can indicate whether a reaction will occur spontaneously (exothermic) or not (endothermic).
- Understanding reaction mechanisms:The sign of the equilibrium constant can provide insights into the reaction mechanism and the relative stability of the reactants and products.
- Designing chemical processes:By manipulating the sign combinations of reactions, chemists can design chemical processes that are more efficient and less energy-intensive.
Case Studies
A case study of the Haber process, which synthesizes ammonia from nitrogen and hydrogen, demonstrates the application of sign combination classification. The overall reaction is exothermic, with a positive sign, indicating that it will occur spontaneously. The equilibrium constant is also positive, indicating that the reaction favors the formation of ammonia.
Future Directions: Classify The Possible Combinations Of Signs For A Reactions
Emerging trends in sign combination classification include the development of computational methods for predicting sign combinations and the use of artificial intelligence to analyze large datasets of reaction data. These advancements will further enhance our understanding of chemical reactions and their applications.
FAQ Compilation
What is the significance of classifying sign combinations for reactions?
Classifying sign combinations for reactions enables chemists to decipher the underlying patterns and relationships between signs and reactions, facilitating the prediction and comprehension of chemical behavior.
How does sign combination classification aid in understanding reactions?
By categorizing sign combinations, chemists can identify commonalities and differences among reactions, allowing for the development of generalized principles that govern chemical reactivity.
What are the practical applications of sign combination classification?
Sign combination classification finds applications in various fields, including drug design, materials science, and environmental chemistry, where it guides the development of new compounds and materials with desired properties.