Electron configuration worksheet and answers provide an invaluable tool for students and educators to understand the fundamental principles of electron configuration. This guide delves into the purpose, types, and examples of electron configuration worksheets, offering a comprehensive resource for mastering this essential chemistry concept.
Electron configuration worksheets empower learners to determine the electron distribution of elements, a crucial aspect of understanding chemical bonding, reactivity, and various properties of matter. By working through these worksheets, students can reinforce their grasp of the Aufbau principle, periodic trends, and the significance of electron configuration in chemistry.
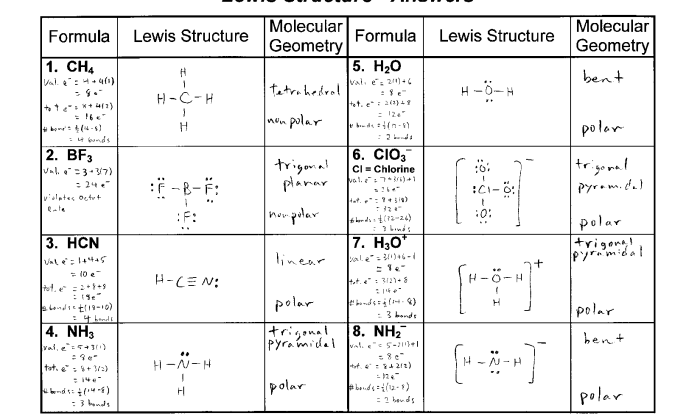
Electron Configuration Worksheet
An electron configuration worksheet is a tool used to help students understand the electron configuration of atoms. Electron configuration refers to the arrangement of electrons in different energy levels or orbitals around the atom’s nucleus.
Electron configuration worksheets typically provide a table or diagram that students can use to fill in the electron configuration of various elements. This helps them visualize the distribution of electrons and understand the periodic trends in electron configuration.
Types of Electron Configuration Worksheets
- Basic Electron Configuration Worksheets:These worksheets focus on the basic principles of electron configuration and provide simple exercises for students to practice.
- Advanced Electron Configuration Worksheets:These worksheets cover more complex concepts, such as Hund’s rule and the Aufbau principle, and provide more challenging exercises.
- Interactive Electron Configuration Worksheets:These worksheets use interactive simulations or online tools to allow students to explore electron configuration in a hands-on way.
Examples of Electron Configuration Worksheets
- Khan Academy Electron Configuration Worksheet
- Education.com Electron Configuration Practice Worksheet
- WorksheetWorks Electron Configuration Worksheet
Electron Configuration Worksheet Answers
Solving electron configuration worksheet problems involves understanding the rules and principles of electron configuration. Here are the steps to follow:
- Determine the atomic number of the element:This is the number of protons in the nucleus, which also equals the number of electrons in a neutral atom.
- Use the periodic table to find the element’s group and period:This will help you determine the number of valence electrons and the energy levels available.
- Follow the Aufbau principle:Electrons fill orbitals in order of increasing energy. Start with the lowest energy level (1s) and fill each orbital with two electrons before moving to the next energy level.
- Apply Hund’s rule:When filling orbitals with the same energy, electrons first occupy separate orbitals with parallel spins before pairing up.
- Write the electron configuration:Use the notation ns xto represent the number of electrons (x) in each energy level (n).
Common Mistakes to Avoid
- Not following the Aufbau principle and filling higher energy levels before lower energy levels.
- Not applying Hund’s rule and pairing electrons before filling all orbitals with one electron.
- Ignoring the number of valence electrons and filling orbitals incorrectly.
- Using the wrong notation or format for the electron configuration.
Electron Configuration
Electron configuration is the distribution of electrons in different energy levels or orbitals around the atom’s nucleus. It is a fundamental property of an element that influences its chemical behavior and properties.
Aufbau Principle, Electron configuration worksheet and answers
The Aufbau principle states that electrons fill orbitals in order of increasing energy. The energy of an orbital is determined by its shape, size, and proximity to the nucleus.
Periodic Table
The periodic table is arranged based on the electron configurations of the elements. Elements in the same group have the same number of valence electrons, which are the electrons in the outermost energy level. Valence electrons determine an element’s chemical reactivity.
Electron Configuration Examples: Electron Configuration Worksheet And Answers
- Hydrogen (H):1s 1
- Helium (He):1s 2
- Lithium (Li):1s 22s 1
- Carbon (C):1s 22s 22p 2
- Oxygen (O):1s 22s 22p 4
Significance of Electron Configuration
Electron configuration plays a crucial role in chemistry as it:
- Determines an element’s chemical reactivity
- Predicts the formation and stability of chemical bonds
- Explains the periodic trends in chemical properties
- Provides insights into the electronic structure and properties of materials
FAQ
What is the purpose of an electron configuration worksheet?
Electron configuration worksheets provide a structured and guided approach for students to practice determining the electron distribution of elements, reinforcing their understanding of the Aufbau principle and periodic trends.
How can I solve electron configuration worksheet problems effectively?
To solve electron configuration worksheet problems effectively, follow these steps: identify the atomic number of the element, determine the number of electrons based on its position in the periodic table, and distribute the electrons according to the Aufbau principle, filling orbitals in order of increasing energy.
What are some common mistakes to avoid when solving electron configuration worksheet problems?
Common mistakes to avoid include: not following the Aufbau principle, exceeding the maximum number of electrons in each orbital, and neglecting to account for the number of valence electrons.